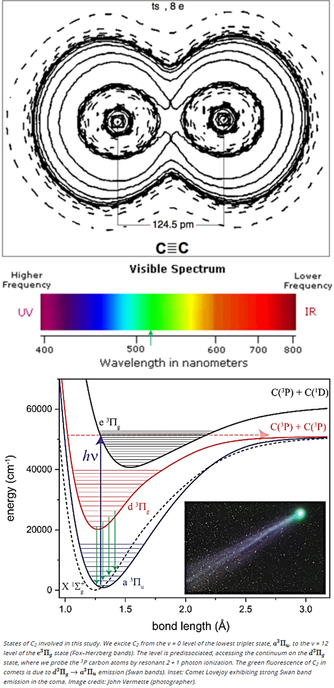
The green color seen in the coma of most comets, but not in their tails, is due to emissions from Diatomic carbon C2 (aka dicarbon) molecules.
Sunlight heats the comet’s ice and organic material to produce C2 molecules, which break apart in ~2 days before they reach the tail. C2 is excited by solar UV radiation and emits mostly in infrared but its triplet state radiates at 518 nm (d3Πg → a3Πu transition below).
https://physicstoday.scitation.org/do/10.1063/pt.6.1.20220110a/full/
https://www.pnas.org/doi/10.1073/pnas.2113315118
#dicarbon #PonsBrooks
5/n