Celltrion Co. advanced its global expansion strategy at BIO USA 2025 in Boston, holding over 150 meetings to strengthen partnerships and pipeline, as it targets growth into a global big pharma.
#YonhapInfomax #Celltrion #BIOUSA #GlobalPartnerships #Biosimilar #AntibodyDrugConjugate #Economics #FinancialMarkets #Banking #Securities #Bonds #StockMarket
https://en.infomaxai.com/news/articleView.html?idxno=68476
#biosimilar
https://www.alojapan.com/1293640/samsung-bioepis-partners-with-nipro-to-commercialize-biosimilars-in-japan-world-business-outlook/ Samsung Bioepis Partners with NIPRO to Commercialize Biosimilars in Japan » World Business Outlook #agreement #biosimilar #Biosimilars #business #Collaborating #footprint #healthcare #innovation #investment #Japan #JapanNews #license #medicines #news #partnership #samsung #SB17 #technology #ustekinumab #WBO #WorldBusinessOutlook Samsung Bioepis Co., Ltd., on June 8th, announced that the company has entered into a license, development and comm…
Samsung Bioepis has dismissed speculation over a potential dual listing, as Samsung Biologics announces a spin-off to separate its CDMO and biosimilar businesses, emphasizing that an IPO for Bioepis is not under consideration.
#YonhapInfomax #SamsungBioepis #SamsungBiologics #SpinOff #CDMOBusiness #Biosimilar #Economics #FinancialMarkets #Banking #Securities #Bonds #StockMarket
https://en.infomaxai.com/news/articleView.html?idxno=64634
Today in #Employer Coverage: an academic study suggests that the #biosimilar market is working, but employers continue to pay high prices as patents expire on brand name biologic medications.
https://open.substack.com/pub/employercoverage/p/biosimilars-are-finally-widely-available
Despite layoff announcements, Novartis beats Q2 expectations — GoodRx teams with Boehringer on Humira biosimilar— Medtech firms have cut more than 14,000 jobs in the past 18 months —http://bit.ly/w28kSd #novartis #earnings #layoffs #humira #biosimilar #goodrx #abbvie #medtech #pharma #pharmanews #biotech #biopharma #biotechnology #cafepharma
FDA OKs First Interchangeable, High-Concentration Humira Biosimilar
https://www.medscape.com/viewarticle/fda-oks-first-interchangeable-high-concentration-humira-2024a10003rd?src=rss&form=fpf #medicine #biosimilar #humira #adalimumab #Simlandi #antirheumatic
EMA Approves Treatments for Psoriasis and Other Autoimmune Disorders
https://www.medscape.com/viewarticle/ema-approves-treatments-psoriasis-and-other-autoimmune-2024a10003nw?src=rss #EMA #Treatments #Psoriasis #Autoimmune #Disorders #biosimilar #Pyzchiva #apremilast
Treatment
For MS now viable
Tyruko, a biosimilar
Providing a cost-effective
Alternative
#fda #ms #tyruko #biosimilar #tysabri #cinquain #poetry
http://www.medicalnewstoday.com/articles/fda-approves-first-natalizumab-biosimilar-for-ms-treatment
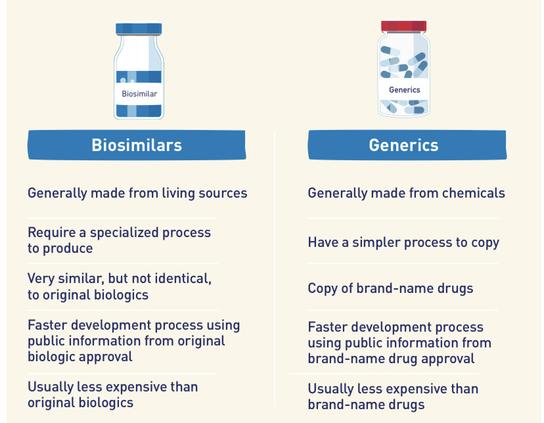
Happy #PharmPhriday! A question I get a lot is “what’s a #biosimilar?” Well, it’s like a #generic but for #biologics
Because a biologic is made from something living, you can’t exactly copy the drug recipe like you can with generics. There are also some differences in testing.
A biosimilar has no clinically meaningful differences from an existing FDA-approved biologic.
A generic drug demonstrates bioequivalence to an existing FDA-approved drug.
Infographic from https://www.fda.gov/media/166334/download
📔 @epiphare publie un article dans la revue BioDrugs
🔎 Severe Hypersensitivity Reactions at #Biosimilar versus Originator #Rituximab Treatment Initiation, Switch and Over Time #EpiVerse
💡 Cette étude ne permet pas de conclure à une association entre exposition aux biosimilaires du Rituximab et hospitalisation pour choc anaphylactique ou réaction sérique à la suite de l’injection, aussi bien à l’initiation, au switch, et au cours du temps.
👉 https://www.epi-phare.fr/rapports-detudes-et-publications/rituximab-hypersensibilite/
Which #adalimumab #biosimilar has #interchangeability status?
💎To learn more what all the new Biosimilar mean to your #IBD patients & practice join our Q/A w @GI_PharmD @DCharabaty 👇🏽👇🏽
#IBDPoll1️⃣
🆓#CME👉🏼 http://bit.ly/3ioJwmM
39 y/o #IBD in clinical & endo remission on adalimumab 40 mg SQ Q2 wks.
Receives a letter from her insurance that she needs to #switch to ADAL #biosimilar and she contacts your office.
How do you proceed?
(RP=reference product)
oh thank you 🥰. it's not looking like it's designed for mobile so will try on desktop.
thank you for jump starting me on trying to understand #biologic v. #biosimilar
reading this now to learn more.
https://www.medicalnewstoday.com/articles/biologics-and-biosimilars#summary
Initiative in Congress aims to improve access to biosimilars
https://www.fiercepharma.com/pharma/proposal-congress-would-eliminate-interchangeable-status-biosimilars #biosimilar #pharma
Do you know what a #biosimilar #medicine is ❓
To date EMA has approved 8⃣6⃣ biosimilars that help treating serious diseases such as #diabetes and #cancer.
All EU-approved 🇪🇺 biosimilars are interchangeable with their reference medicine or an equivalent biosimilar ✅
RT @EMA_News: Do you know what a #biosimilar #medicine is ❓
To date EMA has approved 8⃣6⃣ biosimilars that help treating serious diseases such as #diabetes and #cancer.
All EU-approved 🇪🇺 biosimilars are interchangeable with their reference medicine or an equivalent biosimilar ✅
RT @EMA_News: 🆕 EU-approved 🇪🇺 #biosimilar #medicines are interchangeable with their reference medicine or an equivalent biosimilar 💊💉
Biosimilar medicines provide #patients with more therapeutic options to treat serious diseases such as #cancer and #diabetes.
👉 https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged
🆕 EU-approved 🇪🇺 #biosimilar #medicines are interchangeable with their reference medicine or an equivalent biosimilar 💊💉
Biosimilar medicines provide #patients with more therapeutic options to treat serious diseases such as #cancer and #diabetes.
👉 https://www.ema.europa.eu/en/news/biosimilar-medicines-can-be-interchanged
Proposed Rule Designed to Open #Biosimilar Competition. https://lnkd.in/fFrPHte