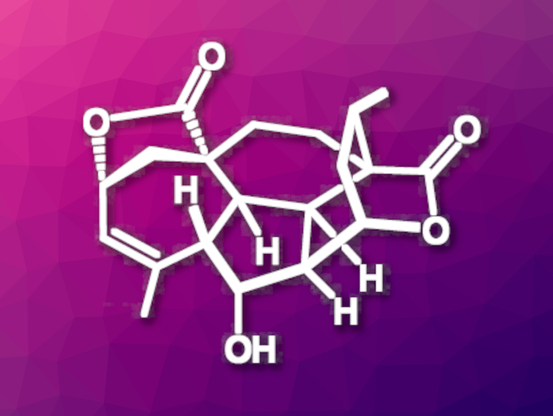
Novel enantioselective #synthesis of rubriflordilactone B by Gui and colleagues from the Chinese Academy of Science published in #JACS
This is already the second total synthesis of this group, with an interesting reaction cascade as the key reaction in this process.
#totalsynthesis
Joseph Tuccinardi and John Wood from Baylor University (#USA), introduced a novel strategy to efficiently synthesize the core scaffold of aleutianamine. Their study involved a vinylogous Mukaiyama-Michael reaction cascade using two previously unknown coupling partners. Published in #JACS
https://me.organicchemistry.eu/post/aleutianamine-jacs-2025/
Computer-aided synthesis: R. Shenvi and C. Li published in #Nature the total synthesis of 25 picrotoxanes. Their 1,5-HAT key reaction was challenged by β-scission, therefore DFT based calculations helped to design usable intermediates.
Read more on my page: https://me.organicchemistry.eu/post/picrotoxanes-nature-2024/
Total Synthesis of Kasugamycin:
Aminoglycoside antibiotic prepared from naturally derived carbohydrates
https://www.chemistryviews.org/total-synthesis-of-kasugamycin/
#orgchem #glycosides #totalsynthesis #chemistry #chemistryviews #chemviews
Total Synthesis of (+)-Mannolide B
Path to a natural product with a complex, fused hexacyclic scaffold
https://www.chemistryviews.org/total-synthesis-of-mannolide-b/
#totalsynthesis #naturalproducts #chemistry #chemistryviews #chemviews
Another total synthesis in the spotlight: Already early this year Mingji Dai and his research team described the total synthesis of heilonine in #JACS (doi: 10.1021/jacs.3c13492). This synthesis is short and elegant, using multiple C-H functionalisations and a Nazarov cyclisation for their key step.
Total Synthesis of Pleurotin (JACS Au, Y. Q. Long 2024)
Pleurotin is a benzoquinone meroterpenoid that was first isolated in the late 1940s. Since that time, several total syntheses of this compound have been reported. Recently, Ya-Qiu Long and his team from Soochow University (#China) published a novel synthetic route in the journal JACS Au.
Read more here: https://www.organicchemistry.eu/books/total-syntheses-of-natural-products/page/total-synthesis-of-pleurotin-y-q-long-2024
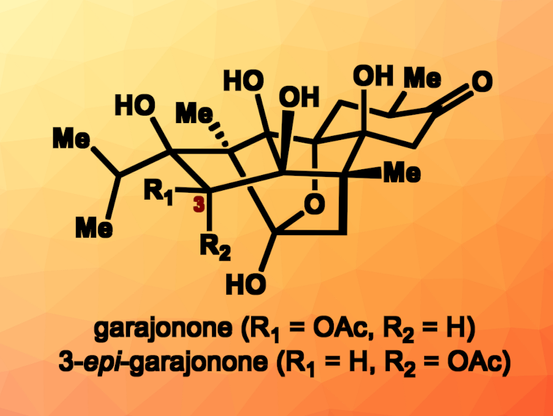
First Total Synthesis of Garajonone
Two-stage synthetic strategy provides access to ryanodane diterpenes, a type of structurally complex natural product
https://www.chemistryviews.org/first-total-synthesis-of-garajonone/
#naturalproducts #totalsynthesis #chemistry #chemistryviews #chemviews
Stasseriolides are a class of polyketide natural products with interesting biological activities, but they are limited in natural abundance. A recent synthesis of stasseriolides A-D by Alois Fürstner's group at the Max Planck Institute für Kohlenforschung reported in Angewandte Chemie employed ring-closing alkyne metathesis, providing access to the natural products and enabling the preparation of analogues.
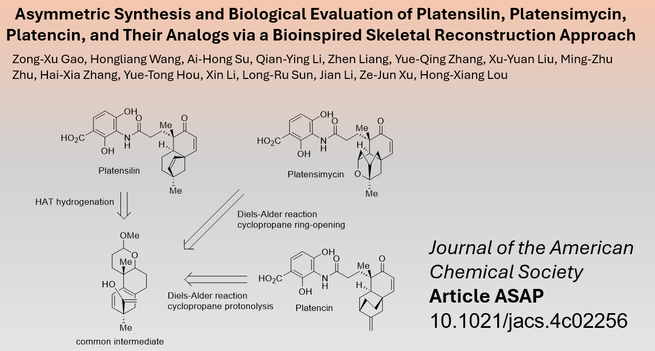
Terpenoids are a large class of secondary #NaturalProducts, with some possessing an interesting polycyclic core that has attracted research interest. Researchers from #Shandong #University in #China led by Ze-Jun Xu (徐泽军) and Hong-Xiang Luo (娄红祥) have now reported the #TotalSynthesis of platensilin and platensimycin, as well as the formal synthesis of platencin, using a biomimetic approach from a common precursor.
Read more in #JACS: https://pubs.acs.org/doi/10.1021/jacs.4c02256
Solvent-Promoted Total Syntheses of Sesquiterpenoid Dimers
Gochnatiolide D and ainsliadimer A prepared via a one-pot sequence using hexafluoroisopropanol (HFIP) as the solvent
https://www.chemistryviews.org/solvent-promoted-total-syntheses-of-sesquiterpenoid-dimers/
#totalsynthesis #hfip #sesquiterpenoids #orgchem #chemistry #chemistryviews
Total Synthesis of (+)-Lemnardosinane A
A sesquiterpenoid natural product isolated from marine soft corals
https://www.chemistryviews.org/total-synthesis-of-lemnardosinane-a/
#totalsynthesis #organicchemistry #orgchem #chemistry #chemistryviews
My Reading Tip of the Week:
Scabrolide A, a polycyclic marine natural product, has fascinated chemists due to its highly constrained structure. Recently, Pengfei Hu and colleagues from Westlake University in Hangzhou, China, published an innovative radical cascade strategy for efficiently synthesizing the scaffold of this polycyclic compound.
Divergent Synthesis of Scabrolide A and Havellockate via an exo-exo-endo Radical Cascade
Researchers (Rahul Choudhury et al.) from the Academy of Scientific and Innovative Research in #India reported in the journal #OrganicLetters the total synthesis of benzo[g]isochromene stereodiads. Their synthesis using a TiCl4 promoted #Aldol coupling showed that the previously reported absolute structure did not agree with the structure of the natural product and a revision was suggested.
Melognine was thought to be a highly complex alkaloid with a polycyclic ring system. Yui Irie and Satoshi Yokoshima (Nagoya University, #Japan) published a total synthesis of the proposed structure in the journal #JACS. However, the synthesised structure turned out to have different analytical properties. The authors concluded that melognine is actually identical to melodinine L. Check out the intriguing total synthesis route on JACS:
https://pubs.acs.org/doi/10.1021/jacs.4c02086
If you have some time, take a look at the total synthesis of Hunterine A by Elliot Hicks, Kengo Inoue and Brian Stoltz published in #JACS (#OpenAccess). Very elegant synthesis and late-stage cyclization strategy.
First Total Synthesis of Pepluacetal
Route relying on a Wolff rearrangement/lactonization cascade, a ring-closing metathesis/cyclopropanation sequence, and a late-stage transannular carbenoid insertion
https://www.chemistryviews.org/first-total-synthesis-of-pepluacetal/
@angew_chem #chemistry #chemistryviews #chemviews #chemivers #Research #TotalSynthesis #organicchemistry #angewandtechemie
Paper out! Only 7.5 years after defending my theses. Finally I can close that chapter of my life.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202315423
#TotalSynthesis #naturalproducts
⚗️ Using the Bunsen burner for the final step of a complex total synthesis?
📰 #OpenAccess #TotalSynthesis of Euphorikanin A by Erick Carreira and colleagues from #ETH Zurich (#Switzerland) published in #JACS. A late-stage atrospecific cascade reaction led to the core structure of the terpene and pyrrolysis finally yielded the natural product.
First Total Syntheses of Hyperireflexolides A and B: Bioinspired pathway involves dearomatization and fragmentation of an acylphloroglucinol
#chemistry #chemistryviews #chemviews #chemiverse #totalsynthesis #organicchemistry #research