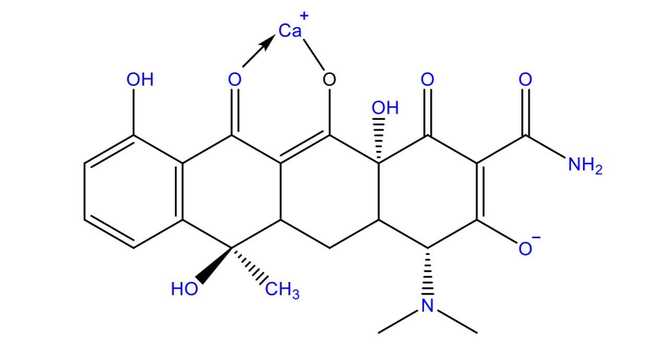
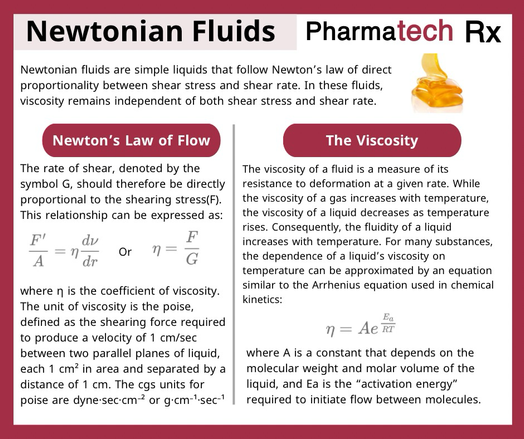
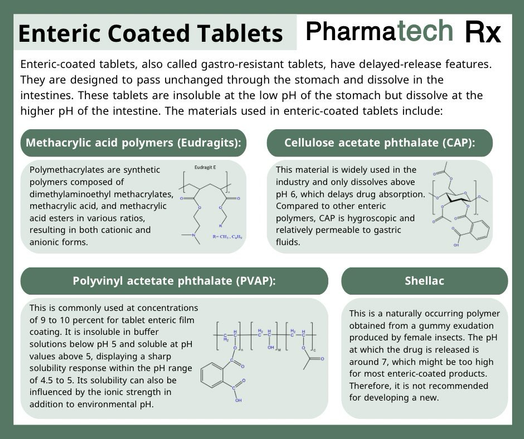
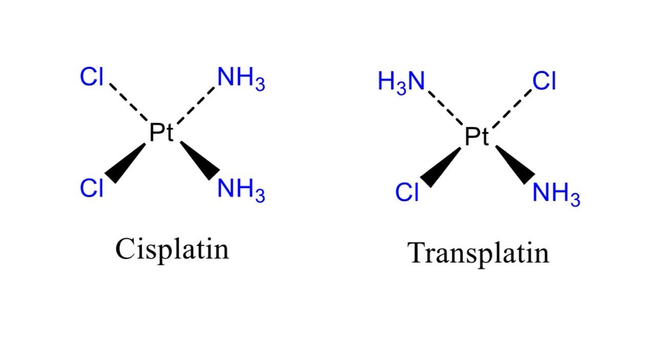
This equation is known as the general ideal gas law, and because it relates the specific conditions or state, that is, the pressure, volume, and temperature of agiven mass of gas, it is called the equation of state of an ideal gas. Real gases do not interact without energy exchange, and therefore do not follow the laws of Boyle and of Gay-Lussac and Charles as ideal gases are assumed to do.
The article: https://pharmatech-rx.com/ideal-gas/