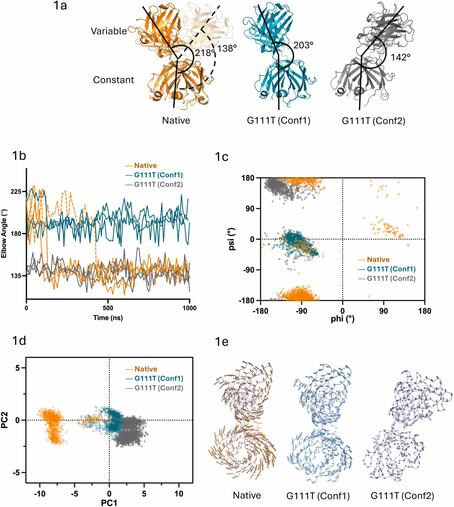
'Clinical trial underway to assess effectiveness and safety of potential Long Covid treatment'
"A clinical trial is underway to assess the effectiveness and safety of sipavibart, AstraZeneca's long-acting monoclonal antibody designed to provide protection against Covid-19, as a potential treatment for Long Covid"
"The study will test whether the monoclonal antibody sipavibart, which is approved for the pre-exposure prophylaxis (prevention) of COVID-19 in Japan and the EU, is effective in treating Long Covid. The trial, which the FDA reviewed and cleared for the study earlier this year, is one of three Long Covid treatment trials expected to begin in 2025 that have been initiated and funded by SILC, a nonprofit organization founded in 2023 by philanthropists Eric and Wendy Schmidt to advance Long Covid care for patients globally."
"In 2024, researchers from the state published a study in the American Journal of Emergency Medicine detailing how a small group of these patients' Long COVID symptoms disappeared after they received the monoclonal antibodies to prevent or treat acute episodes of the virus."
"double-blind, randomized and controlled trial"
https://www.news-medical.net/news/20250501/Clinical-trial-underway-to-assess-effectiveness-and-safety-of-potential-Long-Covid-treatment.aspx
@longcovid
#health #LongCovid #sipavibart #MonoclonalAntibodies